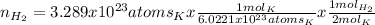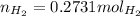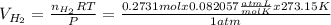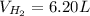Chemistry, 01.11.2019 05:31 lkarroum3733
If 3.289 x 1023 atoms of potassium react with excess water, how many liters of hydrogen gas would be produced at stp? (hint: watch significant figures and rounding.)
2 k + 2 h2o à 2 koh + h2

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Based on the law of conservation of energy, which statement is false? answer- energy is lost when machines dont work right
Answers: 1

Chemistry, 21.06.2019 20:30
18. use the activity series to predict whether the following synthesis reaction will occur. write the chemical equations for the reaction if it's predicted to occur. (s) + o2(g) -> *note: it is possible.*
Answers: 1

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 07:00
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2
You know the right answer?
If 3.289 x 1023 atoms of potassium react with excess water, how many liters of hydrogen gas would be...
Questions

Mathematics, 05.09.2020 22:01

Mathematics, 05.09.2020 22:01

English, 05.09.2020 22:01

Chemistry, 05.09.2020 22:01




Mathematics, 05.09.2020 22:01







Mathematics, 05.09.2020 22:01



History, 05.09.2020 22:01


Advanced Placement (AP), 05.09.2020 22:01