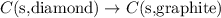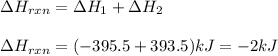Chemistry, 11.01.2020 03:31 mallorybranham
Given the equations below, which description applies to the conversion of diamond to graphite? c(s, diamond) + o2 (g) → co2 (g), ∆h = –395.4 kj co2 (g) → c(s, graphite) + o2 (g), ∆h = 393.5 kj explain your answer
a) energy is created during the process.
b) heat is neither released nor absorbed during the process.
c) heat is released during the process.
d) the process is endothermic.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 06:30
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

Chemistry, 22.06.2019 21:50
Liquid from a brewery fermentation contains 10% ethanol and 90% water. part of the fermentation product (50,000 kg/h) is pumped to a distillation column on the factory site. under current operating conditions, a distillate of 45% ethanol and 55% water is produced from the top of the column at a rate of one-tenth that of the feed. what is the composition of the waste "bottoms" from the still?
Answers: 2
You know the right answer?
Given the equations below, which description applies to the conversion of diamond to graphite? c(s,...
Questions


Mathematics, 12.01.2021 01:00


Mathematics, 12.01.2021 01:00



Mathematics, 12.01.2021 01:00

Mathematics, 12.01.2021 01:00



Chemistry, 12.01.2021 01:00


Mathematics, 12.01.2021 01:00

Physics, 12.01.2021 01:00


Mathematics, 12.01.2021 01:00



Geography, 12.01.2021 01:00

Mathematics, 12.01.2021 01:00

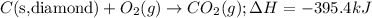 ....(1)
....(1)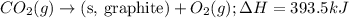 ....(2)
....(2)