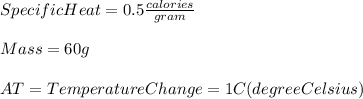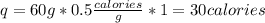Chemistry, 16.11.2019 04:31 fhggggy5680
The specific heat of ice is 0.5 calories/gram. 60 grams of ice will require calories to raise the temperature 1°c. 0.5 30 60 120

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Mrs. smith ordered a root beer float (vanilla ice cream + root beer). mrs. smith noticed that the three states of matter (solid, liquid, and gas) all existed simultaneously in her root beer float. a. identify each phase of matter in the root beer float. b. describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? ) c. identify one phase change you would see in a root beer float and described what causes this change.
Answers: 2



Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1
You know the right answer?
The specific heat of ice is 0.5 calories/gram. 60 grams of ice will require calories to raise the t...
Questions

Chemistry, 04.06.2021 21:00



Business, 04.06.2021 21:00

Mathematics, 04.06.2021 21:00



Mathematics, 04.06.2021 21:00

English, 04.06.2021 21:00



Biology, 04.06.2021 21:00

Mathematics, 04.06.2021 21:00

Mathematics, 04.06.2021 21:00


History, 04.06.2021 21:00

Mathematics, 04.06.2021 21:00