An aqueous solution is 15.0% by mass of copper(ii) sulfate pentahydrate, cuso4∙5h2o. what
is th...


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1

Chemistry, 22.06.2019 19:10
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2

Chemistry, 23.06.2019 01:30
Witch two conditions can limit the usefulness of the kinetic molecular theory in describing gas behavior?
Answers: 2

Chemistry, 23.06.2019 03:50
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer
Answers: 1
You know the right answer?
Questions




English, 18.10.2021 14:30

Mathematics, 18.10.2021 14:30


English, 18.10.2021 14:30

Physics, 18.10.2021 14:30


Biology, 18.10.2021 14:30



English, 18.10.2021 14:30

Mathematics, 18.10.2021 14:30

History, 18.10.2021 14:30

World Languages, 18.10.2021 14:30

English, 18.10.2021 14:30

Mathematics, 18.10.2021 14:30

Mathematics, 18.10.2021 14:30

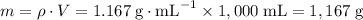 .
.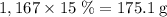 among that 1,167 grams of the solution is
among that 1,167 grams of the solution is 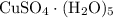 .
. 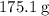 of the hydrate:
of the hydrate: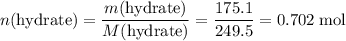 .
.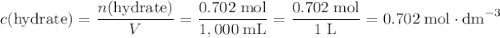 .
.

