
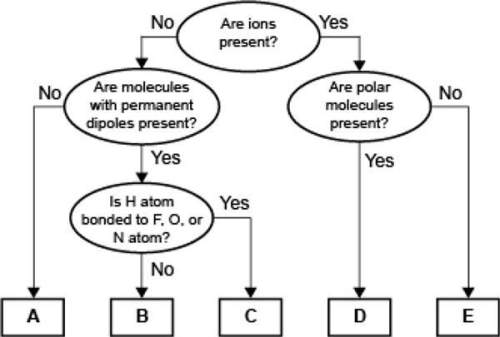
Aconcept map for four types of intermolecular forces and a certain type of bond is shown.
which of the following correctly identifies the intermolecular force represented by a and compares its strength relative to the intermolecular force represented by b?
a represents london dispersion forces, which are weaker than the force represented by b.
a represents hydrogen bonding, which is weaker than the force represented by b.
a represents london dispersion forces, which are stronger than the force represented by b.
a represents hydrogen bonding, which is stronger than the force represented by b.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
What are the charges of the subatomic particles by choosing the answer from the drop down menu. protons have a (+1,0,or-1). (protons,neutrons,electrons) have a 0 charge. 3.) electrons have a (+1,0,-1)
Answers: 2

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 11:00
Problem page combustion of hydrocarbons such as pentane ( c5 h12 ) produces carbon dioxide, a "greenhouse gas." greenhouse gases in the earth's atmosphere can trap the sun's heat, raising the average temperature of the earth. for this reason there has been a great deal of international discussion about whether to regulate the production of carbon dioxide.(a) write a balanced chemical equation, including physical state symbols, for the combustion of liquid pentane into gaseous carbon dioxide and gaseous water. (b) suppose 0.350 kg of pentane are burned in air at a pressure of exactly 1 atm and a temperature of 20.0 degree c. calculate the volume of carbon dioxide gas that is produced.be sure your answer has the correct number of significant digits.
Answers: 2

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1
You know the right answer?
Aconcept map for four types of intermolecular forces and a certain type of bond is shown.
Questions





History, 19.10.2019 14:00

History, 19.10.2019 14:00

Mathematics, 19.10.2019 14:00


Computers and Technology, 19.10.2019 14:00

Mathematics, 19.10.2019 14:00

History, 19.10.2019 14:00


Mathematics, 19.10.2019 14:00

Mathematics, 19.10.2019 14:00

Mathematics, 19.10.2019 14:00


History, 19.10.2019 14:00


History, 19.10.2019 14:00

Biology, 19.10.2019 14:00



