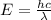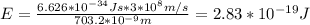Chemistry, 03.02.2020 11:48 tylorroundy
Calculate the energy of the red light emitted by a neon atom with a wavelength of 703.2 nm.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

Chemistry, 22.06.2019 12:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3
You know the right answer?
Calculate the energy of the red light emitted by a neon atom with a wavelength of 703.2 nm....
Questions

History, 22.01.2021 19:10


English, 22.01.2021 19:10

Mathematics, 22.01.2021 19:10







Mathematics, 22.01.2021 19:10

History, 22.01.2021 19:10

Chemistry, 22.01.2021 19:10


Physics, 22.01.2021 19:10

Chemistry, 22.01.2021 19:10