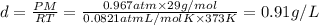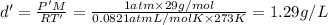Chemistry, 28.11.2019 07:31 Homepage10
When fully inflated a hot air balloon has a volume of 1.6 *10^5 l ( liter) an average temperature of 373k and 0 967 atm . 1) assuming that the air has an average molar mass of 29g/ mol what is the density of the air in the hot air balloon? p = g/l . (2) how does this compare with the density of air at stp ? (a) . it is less dense than at stp . (b) it is more dense than at stp .

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 06:30
Type the correct answer in the box. spell all words correctly.what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3

Chemistry, 22.06.2019 09:00
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1

Chemistry, 23.06.2019 03:00
Abaker touches a pie right after taking it out of the oven. which statement best explains why the pie feels hot?
Answers: 1
You know the right answer?
When fully inflated a hot air balloon has a volume of 1.6 *10^5 l ( liter) an average temperature of...
Questions

Health, 18.10.2020 06:01

Mathematics, 18.10.2020 06:01

Mathematics, 18.10.2020 06:01


Mathematics, 18.10.2020 06:01

Mathematics, 18.10.2020 06:01




English, 18.10.2020 06:01

Social Studies, 18.10.2020 06:01


Mathematics, 18.10.2020 06:01

Mathematics, 18.10.2020 06:01

Chemistry, 18.10.2020 06:01


Mathematics, 18.10.2020 06:01

History, 18.10.2020 06:01


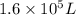
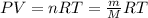
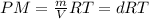
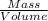 )
)