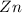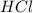When zinc reacts with hydrochloric acid, it produces hydrogen gas. as the reaction proceeds, why does the rate of production of hydrogen gas decrease? the concentration of hydrogen gas decreases. the concentration of the reactants decreases. the hydrogen gas formed inhibits the reaction. the hydrogen gas also reacts with the zinc metal.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
1) this is the structure in the cell nucleus that houses a cell's genetic information
Answers: 3

Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 22.06.2019 13:30
Apush or pull that moves or changes and object when to objects touch
Answers: 2

You know the right answer?
When zinc reacts with hydrochloric acid, it produces hydrogen gas. as the reaction proceeds, why doe...
Questions

Mathematics, 26.09.2019 17:50

English, 26.09.2019 17:50


History, 26.09.2019 17:50


Biology, 26.09.2019 17:50

Spanish, 26.09.2019 17:50

Mathematics, 26.09.2019 17:50


History, 26.09.2019 17:50

Biology, 26.09.2019 17:50

Health, 26.09.2019 18:00


Arts, 26.09.2019 18:00



Biology, 26.09.2019 18:00


Biology, 26.09.2019 18:00

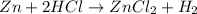
![Rate=k[A]^x[B]^y](/tpl/images/0383/1895/ddde1.png)