
Chemistry, 22.11.2019 06:31 myaaa13754
Find the concentration of h+ ions at a ph = 11 and
ph = 6. then divide the concentration of h+ ions at a
ph = 11 by the of h+ ions at a ph = 6. record your answer in table c.
what is the concentration of h+ ions at a ph = 11?
mol/l
what is the concentration of h+ ions at a ph = 6?
mol/l
how many fewer h+ ions are there in a solution at a
ph = 11 than in a solution at a ph = 6?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2

Chemistry, 22.06.2019 04:00
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3

Chemistry, 22.06.2019 04:50
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1

You know the right answer?
Find the concentration of h+ ions at a ph = 11 and
ph = 6. then divide the concentration of h+...
ph = 6. then divide the concentration of h+...
Questions

Biology, 31.03.2021 07:00

Biology, 31.03.2021 07:00



Mathematics, 31.03.2021 07:00



Mathematics, 31.03.2021 07:00

Mathematics, 31.03.2021 07:00

Mathematics, 31.03.2021 07:00



Mathematics, 31.03.2021 07:00

Mathematics, 31.03.2021 07:00

Mathematics, 31.03.2021 07:00


Mathematics, 31.03.2021 07:00

Mathematics, 31.03.2021 07:00


Mathematics, 31.03.2021 07:00

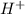 ion concentration.
ion concentration.![pH=-\log[H^+]](/tpl/images/0386/0640/cf945.png)
![11=-\log[H^+]](/tpl/images/0386/0640/c91a3.png)
![[H^+]=1\times 10^{-11} mol/L](/tpl/images/0386/0640/23467.png) ..(1)
..(1)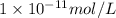 .
.![6=-\log[H^+]'](/tpl/images/0386/0640/502fa.png)
![[H^+]'=1\times 10^{-6} mol/L](/tpl/images/0386/0640/fdc73.png) ..(2)
..(2)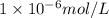 .
.![\frac{[H^+]}{[H^+]'}=\frac{1\times 10^{-11} mol/L}{1\times 10^{-6} mol/L}=1\times 10^{-5}](/tpl/images/0386/0640/2e79f.png)
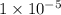 .
.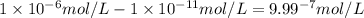
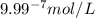 ions fewer than in a solution at a pH = 6
ions fewer than in a solution at a pH = 6

