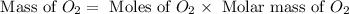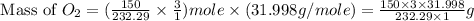Chemistry, 31.01.2020 09:02 gamingisfun
In an experiment, zinc chlorate decomposed according to the following chemical equation.
zn(clo3)2 → zncl2 + o2
(molar mass of zn(clo3)2 = 232.29 g/mol; zncl2 = 136.286 g/mol; o2 = 31.998 g/mol)
if the mass of zinc chlorate was 150 grams, which of the following calculations can be used to determine the mass of oxygen gas formed?
(150 × 1 × 232.29) ÷ (31.998 × 3) grams
(150 × 3 × 232.29) ÷ (31.998 × 1) grams
(150 × 1 × 31.998) ÷ (232.29 × 3) grams
(150 × 3 × 31.998) ÷ (232.29 × 1) grams

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:10
Here’s one way to follow the scientific method. place the missing steps in the correct position in the process
Answers: 1

Chemistry, 22.06.2019 04:30
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3

Chemistry, 22.06.2019 05:40
Why did southern business leaders want to increase the number of slaves
Answers: 1

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1
You know the right answer?
In an experiment, zinc chlorate decomposed according to the following chemical equation.
...
...
Questions

English, 12.01.2021 21:00

Mathematics, 12.01.2021 21:00

Mathematics, 12.01.2021 21:00

English, 12.01.2021 21:00

English, 12.01.2021 21:00



English, 12.01.2021 21:00

Social Studies, 12.01.2021 21:00




Mathematics, 12.01.2021 21:00


Mathematics, 12.01.2021 21:00


Mathematics, 12.01.2021 21:00



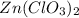
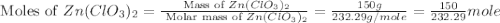
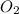
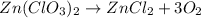
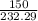 moles of
moles of 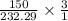 moles of
moles of