

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Which is the layer underground where all empty spaces are filled with a combination of air and water ?
Answers: 1

Chemistry, 22.06.2019 04:30
Use the drop-down menus to answer each question. which runner finished the 100 m race in the least amount of time? which runner stopped running for a few seconds during the race? at what distance did anastasia overtake chloe in the race?
Answers: 1

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 3

Chemistry, 23.06.2019 10:30
Which of the following pairs of elements is most likely to form an ionic compound? a oxygen and fluorine b sodium and aluminum c calcium and chlorine d nitrogen and sulfur
Answers: 1
You know the right answer?
Which of the following would have the highest vapor pressure?
a.
1.0 m sol...
a.
1.0 m sol...
Questions

Mathematics, 10.09.2020 17:01

Mathematics, 10.09.2020 17:01

Mathematics, 10.09.2020 17:01

Mathematics, 10.09.2020 17:01

Spanish, 10.09.2020 17:01

Computers and Technology, 10.09.2020 17:01

Mathematics, 10.09.2020 17:01

Mathematics, 10.09.2020 17:01

Mathematics, 10.09.2020 17:01

English, 10.09.2020 17:01

Mathematics, 10.09.2020 17:01

Mathematics, 10.09.2020 17:01

Mathematics, 10.09.2020 17:01

Social Studies, 10.09.2020 17:01

Mathematics, 10.09.2020 17:01

English, 10.09.2020 17:01

Mathematics, 10.09.2020 17:01

Mathematics, 10.09.2020 17:01

Mathematics, 10.09.2020 17:01

Mathematics, 10.09.2020 17:01

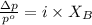
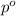 = vapor pressure of the pure component (water)
= vapor pressure of the pure component (water)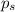 = vapor pressure of the solution
= vapor pressure of the solution 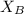 = mole fraction of solute
= mole fraction of solute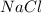 will be,
will be,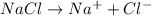
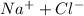 = 1 + 1 = 2
= 1 + 1 = 2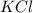 will be,
will be,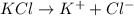
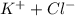 = 1 + 1 = 2
= 1 + 1 = 2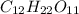 , sucrose is a non-electrolyte solute that means they retain their molecularity, and not undergo association or dissociation.
, sucrose is a non-electrolyte solute that means they retain their molecularity, and not undergo association or dissociation.

