Chemistry, 03.02.2020 08:57 tasnimabdallah971
Energy and specific heat
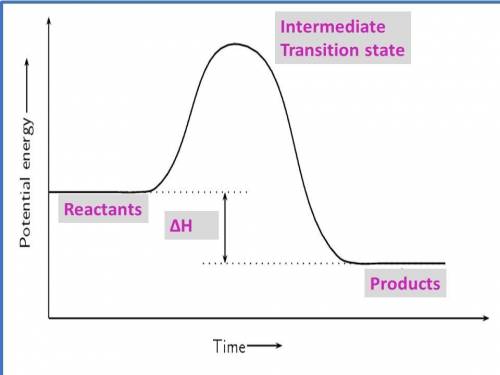
1. draw a graph of an exothermic reaction. label reactants, products and ∆h.
2. calculate the amount of energy required to raise the temperature of 3.00g of gold from 45.9 to 93.0°c.
3. 1.70g of a silvery metal requires 1000.j of energy to change its temp from 298k to 2749k. is the metal pure silver?


Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Which best describes why nh4+ can form an ionic bond with ci-?
Answers: 1

Chemistry, 22.06.2019 22:30
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3

Chemistry, 23.06.2019 00:30
Gasoline has a density of 0.740 g/ml. if you have 328 grams of gasoline, what is the volume in milliliters?
Answers: 1

Chemistry, 23.06.2019 04:31
One student said that the investigation was not valid (a fair test). write a plan for the investigation that includes improvements to the method and apparatus
Answers: 1
You know the right answer?
Energy and specific heat
1. draw a graph of an exothermic reaction. label reactants, prod...
1. draw a graph of an exothermic reaction. label reactants, prod...
Questions





History, 23.02.2021 19:40





Mathematics, 23.02.2021 19:40

Social Studies, 23.02.2021 19:40


Mathematics, 23.02.2021 19:40



Mathematics, 23.02.2021 19:40

Mathematics, 23.02.2021 19:40



Mathematics, 23.02.2021 19:40