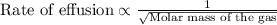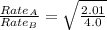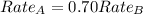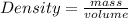Chemistry, 29.09.2019 01:30 zmoore3793
The molar mass of two equally sized samples of unknown gaseous compounds is shown in the table. molar mass comparison gas molar mass a 4.00 g/mol b 2.01 g/mol which statement describes the density and effusion of both gases at stp? gas a has a higher density and effuses faster than gas b. gas a has a higher density and effuses slower than gas b. gas a has a lower density and effuses faster than gas b. gas a has a lower density and effuses slower than gas b.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 17:30
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2

Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1

Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1

Chemistry, 23.06.2019 05:00
Asolution is made by dissolving 2.3 moles of sodium chloride (nacl) in 0.155 kilograms of water. if the molal boiling point constant for water (kb) is 0.51 °c/m, what would be the boiling point of this solution? show all the steps taken to solve this problem.
Answers: 1
You know the right answer?
The molar mass of two equally sized samples of unknown gaseous compounds is shown in the table. mola...
Questions

Mathematics, 28.04.2021 16:30








Mathematics, 28.04.2021 16:30



English, 28.04.2021 16:30

History, 28.04.2021 16:30

Mathematics, 28.04.2021 16:30




Mathematics, 28.04.2021 16:30

Mathematics, 28.04.2021 16:30

History, 28.04.2021 16:30