a. no mass

Chemistry, 23.01.2020 10:31 clevelandjaniya1
Frozen co2 or dry ice is different from co2 gas in the atmosphere because it has
a. no mass
b. a fixed shape
c. a lower density
d. no fixed volume

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

Chemistry, 22.06.2019 09:50
What are four significant sources of ghgs that come from wostem washington?
Answers: 2

Chemistry, 22.06.2019 12:20
Adeuteron, 21h, is the nucleus of a hydrogen isotope and consists of one proton and one neutron. the plasma of deuterons in a nuclear fusion reactor must be heated to about 3.02×108 k . what is the rms speed of the deuterons? express your answer using two significant figures.
Answers: 1
You know the right answer?
Frozen co2 or dry ice is different from co2 gas in the atmosphere because it has
a. no mass
a. no mass
Questions


Chemistry, 17.12.2019 14:31

Mathematics, 17.12.2019 14:31

English, 17.12.2019 14:31



Mathematics, 17.12.2019 14:31


Biology, 17.12.2019 14:31


English, 17.12.2019 14:31

Geography, 17.12.2019 14:31


Mathematics, 17.12.2019 14:31


Social Studies, 17.12.2019 14:31

History, 17.12.2019 14:31

History, 17.12.2019 14:31


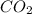 or dry ice is different from
or dry ice is different from 

