
Chemistry, 05.01.2020 14:31 twirlergirl800
Achemistry student weighs out 0.0634g of formic acid hcho2 into a 250.ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1800m naoh solution. calculate the volume of naoh solution the student will need to add to reach the equivalence point. be sure your answer has the correct number of significant digits.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1
You know the right answer?
Achemistry student weighs out 0.0634g of formic acid hcho2 into a 250.ml volumetric flask and dilute...
Questions

English, 26.01.2021 03:10

Social Studies, 26.01.2021 03:10




Computers and Technology, 26.01.2021 03:10

English, 26.01.2021 03:10


Advanced Placement (AP), 26.01.2021 03:10



Mathematics, 26.01.2021 03:10


Mathematics, 26.01.2021 03:10




Mathematics, 26.01.2021 03:10

Chemistry, 26.01.2021 03:10

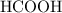 :
: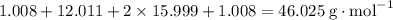 .
.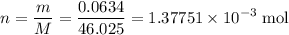 .
.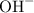 ion to neutralize each carbonyl group
ion to neutralize each carbonyl group 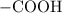 .
.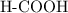 molecule. Each formula unit of NaOH supplies one
molecule. Each formula unit of NaOH supplies one 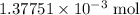 of formic acid in the volumetric flask. It will take the same number of NaOH formula units to reach the equivalence point.
of formic acid in the volumetric flask. It will take the same number of NaOH formula units to reach the equivalence point.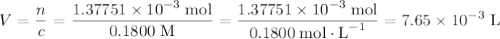 .
.

