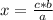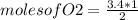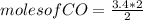Chemistry, 05.01.2020 02:31 katherineedwards1105
Study the reaction.
2co + o2 → 2co2
if 3.4 moles of carbon dioxide (co2) form at the end of the reaction, how many moles of carbon monoxide (co) and oxygen gas (o2) entered the reaction?
2.0 moles of carbon monoxide and 1.0 moles of oxygen gas
6.8 moles of carbon monoxide and 3.4 moles of oxygen gas
3.4 moles of carbon monoxide and 1.7 moles of oxygen gas

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 17:30
Air can be considered a mixture. which statement does not explain why?
Answers: 1

Chemistry, 23.06.2019 03:50
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer
Answers: 1

Chemistry, 23.06.2019 10:30
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 3.75 mol fe and 8.70 mol nio(oh) react?
Answers: 1
You know the right answer?
Study the reaction.
2co + o2 → 2co2
if 3.4 moles of carbon dioxide (co2) for...
2co + o2 → 2co2
if 3.4 moles of carbon dioxide (co2) for...
Questions

Social Studies, 28.08.2020 07:01





English, 28.08.2020 07:01


Mathematics, 28.08.2020 07:01

Mathematics, 28.08.2020 07:01



Mathematics, 28.08.2020 07:01

Mathematics, 28.08.2020 07:01


Mathematics, 28.08.2020 07:01