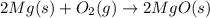Chemistry, 20.11.2019 16:31 reganleigh00
The oxidation of magnesium to form magnesium oxide is shown by which balanced chemical equation?
a) mg(s) + o2(g) → mgo(s)
b) 2mg(s) + o2(g) → 2mgo(s)
c) 2mgo(s) → 2mg(s) + o2(g)
d) 2mg(s) → 2mgo(s) + o2(g)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 12:00
the mississippians were considered to be horticulturalists, which means they were
Answers: 1

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2
You know the right answer?
The oxidation of magnesium to form magnesium oxide is shown by which balanced chemical equation?
Questions

History, 03.09.2021 02:30

Mathematics, 03.09.2021 02:30


History, 03.09.2021 02:30

Mathematics, 03.09.2021 02:30

Computers and Technology, 03.09.2021 02:30

Mathematics, 03.09.2021 02:30


Mathematics, 03.09.2021 02:30

English, 03.09.2021 02:30


Mathematics, 03.09.2021 02:30


English, 03.09.2021 02:30



Mathematics, 03.09.2021 02:30

Mathematics, 03.09.2021 02:30


Mathematics, 03.09.2021 02:30