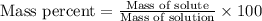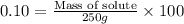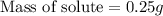Calculate the grams of solute required to make 250 g of 0.10% mgso4 (m/m).
use this form...

Chemistry, 25.11.2019 18:31 picklehead7272
Calculate the grams of solute required to make 250 g of 0.10% mgso4 (m/m).
use this formula: percent by mass: mass of solute/mass of solution×100%

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3


Chemistry, 22.06.2019 19:10
Δu of , in kj/kg, as it isto k, (a)as a of , (b) at , (c) at .
Answers: 2

Chemistry, 23.06.2019 00:30
Five different substances are given to you to be dissolved in water. which substances are most likely to undergo dissolution in water? check all that apply. view available hint(s) check all that apply. sodium fluoride, naf octane, c8h18 propanol, ch3ch2ch2oh potassium iodide, ki benzene, c6h6
Answers: 1
You know the right answer?
Questions


Mathematics, 03.10.2019 07:30


Spanish, 03.10.2019 07:30

Mathematics, 03.10.2019 07:30

History, 03.10.2019 07:30


World Languages, 03.10.2019 07:30






History, 03.10.2019 07:30

English, 03.10.2019 07:30





History, 03.10.2019 07:30

 is 0.25 g
is 0.25 g