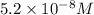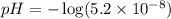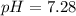1. a solution at 25 degrees celsius is 1.0 × 10–5 m h3o+. what is the concentration of oh– in this solution?
1.0 × 10–5 m oh–
1.0 × 10–14 m oh–
1.0 × 105 m oh–
1.0 × 10–9 m oh–
2.what is the ph of a solution with a concentration of 5.2 × 10–8 m h3o+?
1.01
3.24
7.28
8.72

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:50
The electron configuration for chromium is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 5 4 s 1 instead of 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 4 4 s 1 . the configuration is an exception to the
Answers: 3

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2
You know the right answer?
1. a solution at 25 degrees celsius is 1.0 × 10–5 m h3o+. what is the concentration of oh– in this s...
Questions







History, 26.07.2019 21:00



Mathematics, 26.07.2019 21:00


Biology, 26.07.2019 21:00








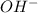 concentration is,
concentration is, 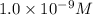
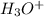 =
= 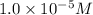
![pH=-\log [H_3O^+]](/tpl/images/0396/5177/841e8.png)
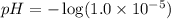
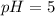
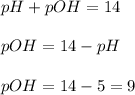
![pOH=-\log [OH^-]](/tpl/images/0396/5177/1fac1.png)
![9=-\log [OH^-]](/tpl/images/0396/5177/1db56.png)
![[OH^-]=1.0\times 10^{-9}M](/tpl/images/0396/5177/ce9a1.png)