
Chemistry, 16.01.2020 21:31 stupidjew5496
In the summer, your tires will increase in volume due to the warm temperatures, so you may need to check your tire pressure. this is an example of
the combined gas law
archimedes' principle
pressure laws
boyle's law

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:00
The earth's moon is unusually large. two popular theories of the moon's origin include the "sister world" hypothesis, which states that the moon formed from the same materials as the earth, near enough to the earth that they fell into orbit around each other. a second theory is the "capture" hypothesis, in which the moon formed elsewhere in the solar system, and the earth's gravity pulled it into its orbit. studies of what the moon is made of indicate that some of its materials had to come from the earth or from the same area of the solar system where the earth had formed. at the same time, the moon does not contain much of the material that makes up the earth's core, so the moon could not have formed from the same materials as the earth. how do the two facts above affect the described theories of the moon's origin? a. they show that scientists will never agree on where the moon came from. b. they show that more experiments on moon formation need to be done. c. they show that no theory accounts for the existence of the moon. d. they show that neither theory is complete and entirely correct.
Answers: 1

Chemistry, 22.06.2019 13:30
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 23:50
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3
You know the right answer?
In the summer, your tires will increase in volume due to the warm temperatures, so you may need to c...
Questions

Computers and Technology, 04.02.2021 21:00



Computers and Technology, 04.02.2021 21:00



History, 04.02.2021 21:00

Mathematics, 04.02.2021 21:00


Medicine, 04.02.2021 21:00

Mathematics, 04.02.2021 21:00



French, 04.02.2021 21:00

Mathematics, 04.02.2021 21:00



English, 04.02.2021 21:00


Mathematics, 04.02.2021 21:00

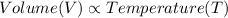 (at constant Pressure)
(at constant Pressure)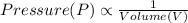 (at constant Temperature)
(at constant Temperature)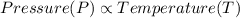 (at constant volume)
(at constant volume)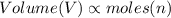 (at constant pressure and temperature)
(at constant pressure and temperature)

