
Chemistry, 28.01.2020 13:40 heyyyyy3922
As with any combustion reaction, the products of combusting a hydrocarbon fuel (cxhy) with oxygen (o2) are carbon dioxide (co2) and water (h2o).
a mass of 15.51 g for an unknown fuel was combusted in a reaction vessel containing an unknown amount of oxygen. at the end of the reaction, there still remained 15.46 g of the fuel as well as 0.0817 g of water and 0.1497 g of carbon dioxide. the oxygen was completely consumed during the reaction.
how many molecules of oxygen gas were initially present in the reaction vessel?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:50
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3

Chemistry, 22.06.2019 05:30
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2

Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

You know the right answer?
As with any combustion reaction, the products of combusting a hydrocarbon fuel (cxhy) with oxygen (o...
Questions

Mathematics, 19.10.2021 14:00



History, 19.10.2021 14:00


Mathematics, 19.10.2021 14:00

Chemistry, 19.10.2021 14:00

Mathematics, 19.10.2021 14:00




Mathematics, 19.10.2021 14:00

Mathematics, 19.10.2021 14:00






Mathematics, 19.10.2021 14:00

Business, 19.10.2021 14:00

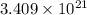
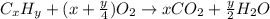
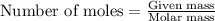
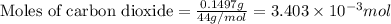
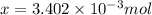
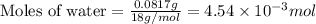
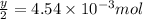
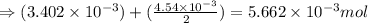
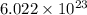 number of molecules.
number of molecules.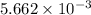 moles of oxygen will contain =
moles of oxygen will contain = 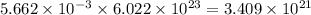 molecules.
molecules.

