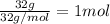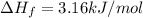Chemistry, 19.11.2019 15:31 Mordred809
How much heat is given off when 32g liquid methanol (ch3oh) at its freezing point changes to solid methanol? (methanol hf=3.16 kj/mol)
a)1.58 kj
b)3.16 kj
c)0.79 kj
d) 0.18 kj

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Which of the following mining methods disrupts the sea floor?
Answers: 1

Chemistry, 22.06.2019 10:10
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2

Chemistry, 23.06.2019 00:30
The molecular weight of carbon dioxide, co2, is 44.00 amu, and the molecular weight of nitrous dioxide, no2, is 46.01 amu, so no2 diffuses co2
Answers: 2

Chemistry, 23.06.2019 01:30
Magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. trial 1: trial 2: data mass of empty crucible with lid trial 1: 26.688 trial 2: 26.681 mass of mg metal, crucible, and lid trial 1: 26.994 trial: 2 26.985 mass of mgo, crucible, and lid trial 1: 27.188 trial 2: 27.180
Answers: 1
You know the right answer?
How much heat is given off when 32g liquid methanol (ch3oh) at its freezing point changes to solid m...
Questions




Social Studies, 28.05.2021 18:10



Mathematics, 28.05.2021 18:10

Mathematics, 28.05.2021 18:10


Social Studies, 28.05.2021 18:20


Mathematics, 28.05.2021 18:20

Spanish, 28.05.2021 18:20


Mathematics, 28.05.2021 18:20


Advanced Placement (AP), 28.05.2021 18:20

Mathematics, 28.05.2021 18:20

Mathematics, 28.05.2021 18:20

Mathematics, 28.05.2021 18:20