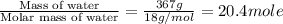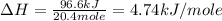Chemistry, 28.09.2019 22:00 emalvidrez5205
The initial temperature of the water in a constant-pressure calorimeter is 24°c. a reaction takes place in the calorimeter, and the temperature rises to 87°c. the calorimeter contains 367 g of water, which has a specific heat of 4.18 j/(g·°c). calculate the enthalpy change (δh) during this reaction

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:20
What are the spectator ions in 2h+ + so42- + ca2+ + 2r → caso4 + 2h+ + 21?
Answers: 1

Chemistry, 22.06.2019 07:00
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1


Chemistry, 22.06.2019 13:30
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2
You know the right answer?
The initial temperature of the water in a constant-pressure calorimeter is 24°c. a reaction takes pl...
Questions

English, 25.03.2021 15:50

English, 25.03.2021 15:50




Biology, 25.03.2021 15:50


Mathematics, 25.03.2021 15:50



Mathematics, 25.03.2021 15:50


Mathematics, 25.03.2021 15:50


Social Studies, 25.03.2021 15:50

Law, 25.03.2021 15:50


Mathematics, 25.03.2021 15:50

Mathematics, 25.03.2021 15:50

Mathematics, 25.03.2021 15:50

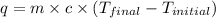
 = specific heat of water =
= specific heat of water = 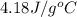
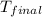 = final temperature of water =
= final temperature of water = 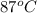
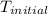 = initial temperature of metal =
= initial temperature of metal = 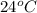
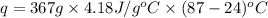
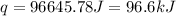
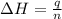
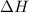 = enthalpy change = ?
= enthalpy change = ?