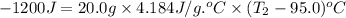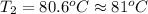Chemistry, 09.10.2019 19:00 daisylaloca
Answer asap! will give brainliest! a pan containing 20.0 grams of water was allowed to cook from a temperature of 95.0 degrees celsius. if the amount of heat released is 1,200 joules, what is the approximate final temperature of the water?
a. 75 degrees celsius
b. 78 degrees celsius
c. 81 degrees celsius
d. 87 degrees celsius

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:30
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1

Chemistry, 22.06.2019 11:00
When hydrochloric acid reacts with potassium hydroxide solution, the following reaction occurs. hcl (aq) + koh (aq) h2o (l) + kcl (aq) the reaction gives off heat energy, so it is an reaction.
Answers: 1

Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1

Chemistry, 22.06.2019 21:20
Phosgene (carbonyl chloride), cocl2, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: co(g) cl2(g)⇌cocl2(g) carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 477 ∘c . at equilibrium, the concentrations were measured and the following results obtained: gas partial pressure (atm) co 0.830 cl2 1.30 cocl2 0.220 what is the equilibrium constant, kp, of this reaction
Answers: 2
You know the right answer?
Answer asap! will give brainliest! a pan containing 20.0 grams of water was allowed to cook from a...
Questions

Mathematics, 06.03.2021 03:50


Mathematics, 06.03.2021 03:50



English, 06.03.2021 03:50

Geography, 06.03.2021 03:50


Mathematics, 06.03.2021 03:50

Biology, 06.03.2021 03:50


Biology, 06.03.2021 03:50

Mathematics, 06.03.2021 03:50

English, 06.03.2021 03:50

Arts, 06.03.2021 03:50



Biology, 06.03.2021 03:50

Mathematics, 06.03.2021 03:50

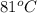
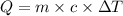
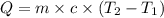
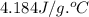
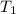 = initial temperature =
= initial temperature = 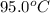
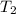 = final temperature = ?
= final temperature = ?