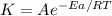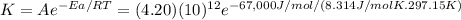Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 17:10
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1

Chemistry, 23.06.2019 01:00
Substance 33°f 100°f peanut oil solid liquid margarine solid liquid chocolate chips solid liquid which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1

Chemistry, 23.06.2019 03:00
Can someone me out on this question for my national 5 chemistry homework
Answers: 1
You know the right answer?
Acertain reaction has an activation energy of 67.0 kj/mol and a frequency factor of a1 = 4.20×1012....
Questions

Mathematics, 16.11.2020 19:50


English, 16.11.2020 19:50

Mathematics, 16.11.2020 19:50


Mathematics, 16.11.2020 19:50



English, 16.11.2020 19:50


Mathematics, 16.11.2020 19:50

Chemistry, 16.11.2020 19:50

Business, 16.11.2020 19:50





Mathematics, 16.11.2020 19:50


Business, 16.11.2020 19:50