
Chemistry, 29.01.2020 14:54 BaileyElizabethRay
Which of the following changes has a decrease in entropy?
co2(s) → co2(g)
2h2o(l) → 2h2(g) + o2(g)
h2o(l) → h2o(s)
2kclo3(s) → 3o2(g) + 2kcl(s)

Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 05:50
Which of the following is not a characteristic of s waves?
Answers: 1

Chemistry, 23.06.2019 06:40
8. how much enthalpy/heat is transferred when 0.5113gof ammonia (nh3) reacts with excess oxygen according| to the following equation: 4nh3 +502 - 4n0+ 6h20ah = -905.4j
Answers: 1

Chemistry, 23.06.2019 12:30
How does a nuclear reactor produce electricity? a. high-energy gamma rays are converted by a generator into electricity. b. the heat from the reaction turns water to steam, which runs a generator. c. the reaction produces a stream of electrons that flow through wires and into batteries. d. the heat released from the reaction is used to burn coal or gas to produce electricity. e. control rods absorb the neutrons emitted and release a stream of electrons as electricity.
Answers: 1

Chemistry, 23.06.2019 18:30
Why must sample spots be above the solvent in paper chromatography
Answers: 1
You know the right answer?
Which of the following changes has a decrease in entropy?
co2(s) → co2(g)
2h2o(l)...
co2(s) → co2(g)
2h2o(l)...
Questions

Biology, 30.11.2019 03:31


Social Studies, 30.11.2019 03:31


Social Studies, 30.11.2019 03:31



Chemistry, 30.11.2019 03:31

Geography, 30.11.2019 03:31

Physics, 30.11.2019 03:31

Biology, 30.11.2019 03:31









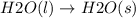 is the change that has a decrease in entropy.
is the change that has a decrease in entropy.

