
One of the main components of an airbag is the gas that fills it. as part of the design process, you need to determine the exact amount of nitrogen that should be produced. calculate the number of moles of nitrogen required to fill the airbag. show your work. assume that the nitrogen produced by the chemical reaction is at a temperature of 495°c and that nitrogen gas behaves like an ideal gas. use this fact sheet to review the ideal gas law.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:40
How many liters of hydrogen gas will be produced at stp from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (h3po4) the equation is 3mg + 2h3(> mg(po4)2+3h2
Answers: 1

Chemistry, 22.06.2019 20:00
State one important difference between a physical change and a chemical change?
Answers: 1

Chemistry, 22.06.2019 23:30
To find the work done, the force exerted and distance moved are multiplied. a couch is moved twice before you are happy with its placement. the same force was used to move the couch both times. if more work is done the first time it is moved, what do you know about the distance it was moved? a) when more work was done, the couch was moved the same distance. b) when more work was done, the couch was moved less. c) when more work was done, the couch was moved further. d) when more work was done, the couch wasn't moved at all.
Answers: 1

Chemistry, 23.06.2019 06:20
An object of mass 10.0 kg and volume 1000 ml and density 10 g/ml sinks in water who’s density is 1.0 g/ml. what is the mass of the water which has been displaced in kilograms
Answers: 1
You know the right answer?
One of the main components of an airbag is the gas that fills it. as part of the design process, you...
Questions


Mathematics, 12.03.2020 02:38





Biology, 12.03.2020 02:38






Mathematics, 12.03.2020 02:38

Spanish, 12.03.2020 02:38






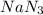 ,
, 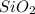 and
and 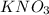 .The temperature at collision increases and
.The temperature at collision increases and 

