

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
When curium-242 is bombarded with an alpha particle, two products are formed, one of which is a nudge on. what is the other product
Answers: 3


Chemistry, 21.06.2019 22:30
Naoki's bicycle has a mass of 10 kg. if naoki sits on her bicycle and starts pedaling with a force of 168 n, causing an acceleration of 2.8 m/s2, what is naoki's mass?
Answers: 1

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1
You know the right answer?
The reaction a + 2b + c -- > d + 2e is first order in reactant a, first order in b, and second or...
Questions

Mathematics, 17.06.2021 19:00



Social Studies, 17.06.2021 19:00

Mathematics, 17.06.2021 19:00

Mathematics, 17.06.2021 19:00

Mathematics, 17.06.2021 19:00


Chemistry, 17.06.2021 19:00

Business, 17.06.2021 19:00

History, 17.06.2021 19:00


Mathematics, 17.06.2021 19:00

Mathematics, 17.06.2021 19:00

Mathematics, 17.06.2021 19:00

History, 17.06.2021 19:00

English, 17.06.2021 19:00



![\text{Rate}=k[A][B][C]^2](/tpl/images/0287/3858/2c5a0.png)
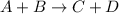
![\text{Rate}=k[A]^a[B]^b](/tpl/images/0287/3858/10aeb.png)
![[A]](/tpl/images/0287/3858/6aa06.png) and
and ![[B]](/tpl/images/0287/3858/db909.png) = concentration of A and B reactant
= concentration of A and B reactant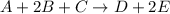
![\text{Rate}=k[A]^1[B]^1[C]^2](/tpl/images/0287/3858/d33f5.png)



