
Chemistry, 28.12.2019 23:31 tagerryawilson6
During a laboratory experiment, a 3.24-gram sample of nahco3 was thermally decomposed. in this experiment, carbon dioxide and water vapors escape and are combined to form carbonic acid. after decomposition, the sample weighed 2.19 grams. calculate the percentage yield of carbonic acid for the reaction. describe the calculation process in detail. nahco3 → na2co3 + h2co3

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Why is the structure of molecule important to its function?
Answers: 1


Chemistry, 23.06.2019 06:30
What is the chemical formula for a compound between li and br? libr li2br libr2 libr3
Answers: 1

Chemistry, 23.06.2019 06:30
Consider the heating curve of h2o and line segments a, b, and c. several changes are taking place at a, b, and c. all but one would be an appropriate description as e move through segments a, b and then c.
Answers: 3
You know the right answer?
During a laboratory experiment, a 3.24-gram sample of nahco3 was thermally decomposed. in this exper...
Questions

Spanish, 13.01.2021 17:50







Mathematics, 13.01.2021 17:50


Mathematics, 13.01.2021 17:50


Mathematics, 13.01.2021 17:50

Mathematics, 13.01.2021 17:50

Computers and Technology, 13.01.2021 17:50






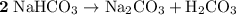 .
.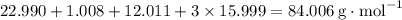 .H₂CO₃:
.H₂CO₃: 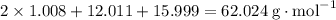 .
.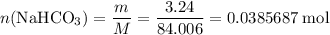 .
.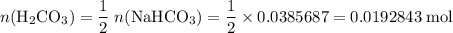
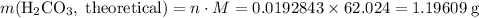 .
.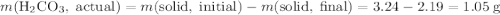 .
. .
.

