Chemistry, 30.01.2020 17:02 xXFLUFFYXx
The following reaction shows sodium hydroxide reacting with sulfuric acid.
naoh + h2so4 → na2so4 + h2o
how many grams of na2so4 are produced from 10.0 grams of naoh?
(molar mass of na = 22.989 g/mol, o = 15.999 g/mol, h = 1.008 g/mol, s = 32.065 g/mol)
17.8 grams
19.2 grams
35.5 grams
38.5 grams

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 15:20
Which description best characterizes the motion of particles in a solid?
Answers: 2

Chemistry, 22.06.2019 16:10
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1

Chemistry, 23.06.2019 06:30
(04.01 lc) which of the following is true about science? (5 points) select one: a. it is not influenced by social conditions. b. it is not determined by external local factors. c. political conditions are unable to influence it. d. economic concerns may prevent it from solving problems.
Answers: 1
You know the right answer?
The following reaction shows sodium hydroxide reacting with sulfuric acid.
naoh + h2so4...
naoh + h2so4...
Questions



Physics, 18.10.2019 18:30

Mathematics, 18.10.2019 18:30


Business, 18.10.2019 18:30


Mathematics, 18.10.2019 18:30

Mathematics, 18.10.2019 18:30


Mathematics, 18.10.2019 18:30




Mathematics, 18.10.2019 18:30


Mathematics, 18.10.2019 18:30



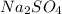 produced will be 17.8 grams.
produced will be 17.8 grams. 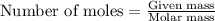 ....(1)
....(1)![NaOH=[(1\times 22.989)+(1\times 15.999)+(1\times 1.008)]g/mol=39.996g/mol](/tpl/images/0486/6119/380bc.png)
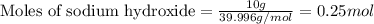
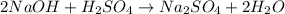
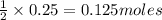 of sodium sulfate.
of sodium sulfate.![Na_2SO_4=[(2\times 22.989)+(1\times 32.065)+(4\times 15.999)]g/mol=142.039g/mol](/tpl/images/0486/6119/878b0.png)