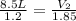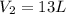Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

Chemistry, 22.06.2019 23:00
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3

Chemistry, 23.06.2019 01:00
If i had 2 m naoh solution, what does the 2 m stand for? 2 molar, but 2 of a solute in 1
Answers: 1
You know the right answer?
A8.5-liter sample of a gas at 2.0 atm and 300.0 k has 1.2 moles of the gas. if 0.65 mole of the gas...
Questions

Mathematics, 01.12.2020 01:00


Mathematics, 01.12.2020 01:00



English, 01.12.2020 01:00

History, 01.12.2020 01:00


Mathematics, 01.12.2020 01:00





Arts, 01.12.2020 01:00

Mathematics, 01.12.2020 01:00


Spanish, 01.12.2020 01:00

English, 01.12.2020 01:00

English, 01.12.2020 01:00

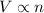 (At constant temperature and pressure)
(At constant temperature and pressure) 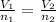
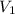 = initial volume of gas = 8.5 L
= initial volume of gas = 8.5 L
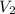 = final volume of gas = ?
= final volume of gas = ?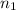 = initial moles of gas = 1.2 moles
= initial moles of gas = 1.2 moles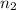 = final moles of gas = 1.2 +0.65 = 1.85 moles
= final moles of gas = 1.2 +0.65 = 1.85 moles