
Chemistry, 21.01.2020 02:31 marquezsturgis
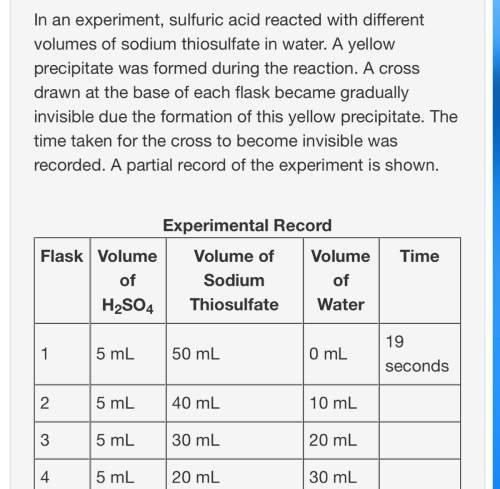
Based on your knowledge of factors that affect the rates of chemical reactions, predict the trend in the last column of the experimental record. use complete sentences to explain the trend you predicted. you do not have to determine exact values for time; just describe the trend you would expect (increase or decrease) and why it occurs
- answer asap! will award brainlist

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
The is a particle with one unit of positive charge a. proton b. positron c. electron d. nucleus awnser quick it is a important science test!
Answers: 2

Chemistry, 22.06.2019 04:00
What three natural resources are found in the great lakes region
Answers: 2

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 15:00
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2
You know the right answer?
Based on your knowledge of factors that affect the rates of chemical reactions, predict the trend in...
Questions

History, 24.10.2019 01:10



History, 24.10.2019 01:10

History, 24.10.2019 01:10

Social Studies, 24.10.2019 01:20


Mathematics, 24.10.2019 01:20

Mathematics, 24.10.2019 01:20


Mathematics, 24.10.2019 01:20

Social Studies, 24.10.2019 01:20

Advanced Placement (AP), 24.10.2019 01:20

Biology, 24.10.2019 01:20

Mathematics, 24.10.2019 01:20


English, 24.10.2019 01:20

History, 24.10.2019 01:20


Computers and Technology, 24.10.2019 01:20



