
Chemistry, 29.01.2020 04:49 kimlyn58p0wyn0
If a given power plant released so2 gas with a volume v of 1200 m3 at a density ρ of 2.86 kg/m3 at standard pressure and temperature, how many moles n of so2 are released? the atomic weight of sulfur is 32.07 u and the atomic weight of oxygen is 16.00 u.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What is the nature of the ca-cl bond in a molecule of calcium chloride (cacl2) if the electronegativity value of calcium is 1.0 and that of chlorine is 3.16?
Answers: 1

Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1

Chemistry, 22.06.2019 14:30
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1

Chemistry, 22.06.2019 23:00
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2
You know the right answer?
If a given power plant released so2 gas with a volume v of 1200 m3 at a density ρ of 2.86 kg/m3 at s...
Questions


Engineering, 13.06.2021 20:30

Mathematics, 13.06.2021 20:30





Computers and Technology, 13.06.2021 20:30

Mathematics, 13.06.2021 20:30

History, 13.06.2021 20:30



Social Studies, 13.06.2021 20:30



Arts, 13.06.2021 20:30


English, 13.06.2021 20:30

Social Studies, 13.06.2021 20:30

Mathematics, 13.06.2021 20:30

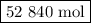
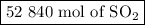 .
.


