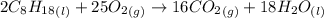Chemistry, 26.08.2019 21:00 ilovebeans25423
The combustion of octane, c8h18, proceeds according to the reaction
2c8h18(l)+25o2(> 16co2(g)+18h2o(l)
if 297 mol of octane combusts, what volume of carbon dioxide is produced at 20.0 °c and 0.995 atm?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
When determining the shape of a molecule, it is important to draw a lewis dot structure first in order to see the total number a. electrons within the moleculeb. bonding and unshared pairs around central atomc. unshared pair within the molecule( i really need it )
Answers: 1

Chemistry, 22.06.2019 00:40
During which time interval does the object travel approximately 10 meters
Answers: 3

Chemistry, 22.06.2019 02:30
24 points and brainliest to anyone who can answer under 10 minutes with best ! the table below shows the role of different substances during photosynthesis. substance role during photosynthesis glucose stores chemical energy water combines with glucose to form carbon dioxide chlorophyll traps sunlight which of the following statements would correct one of the roles listed in the table? glucose combines with carbon to form water. chlorophyll reacts with light to produce carbon dioxide. water combines with carbon dioxide during photosynthesis. chlorophyll stores chemical energy needed for photosynthesis.
Answers: 1

Chemistry, 22.06.2019 05:50
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2
You know the right answer?
The combustion of octane, c8h18, proceeds according to the reaction
2c8h18(l)+25o2(> 16co2(...
2c8h18(l)+25o2(> 16co2(...
Questions



Mathematics, 18.12.2021 14:00

Social Studies, 18.12.2021 14:00


Computers and Technology, 18.12.2021 14:00

Mathematics, 18.12.2021 14:00



Health, 18.12.2021 14:00


Social Studies, 18.12.2021 14:00


History, 18.12.2021 14:00

History, 18.12.2021 14:00




Business, 18.12.2021 14:00