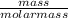Chemistry, 02.11.2019 23:31 pgjohnston001
A55.0g sample of iron (iii) filings is reacted with 23.8g of powdered sulfur (s8). how much iron (iii) sulfide in moles would be produced in this reaction?
equation:
convert to moles
of iron:
convert to moles
of sulfur:
calculate the
limiting reagent:
solve the problem:

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Chemistry, 22.06.2019 18:40
What is one real world example of a colligative property?
Answers: 2

Chemistry, 22.06.2019 18:50
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1

Chemistry, 23.06.2019 03:30
Astudent uses universal ph paper to find the ph of three solutions . solution a has a ph of 5 solution b has a ph of 11 and solution c has a ph of 7 identify which solution is acidic which solution is neutral and which solution is basic
Answers: 1
You know the right answer?
A55.0g sample of iron (iii) filings is reacted with 23.8g of powdered sulfur (s8). how much iron (ii...
Questions

Mathematics, 03.09.2020 19:01

English, 03.09.2020 19:01


Biology, 03.09.2020 19:01

Mathematics, 03.09.2020 19:01

Biology, 03.09.2020 19:01

History, 03.09.2020 19:01

Social Studies, 03.09.2020 19:01

Mathematics, 03.09.2020 19:01

Mathematics, 03.09.2020 19:01

Mathematics, 03.09.2020 19:01






English, 03.09.2020 19:01