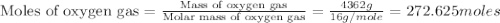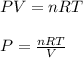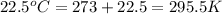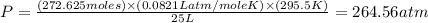Chemistry, 22.09.2019 19:20 glocurlsprinces
Using the ideal gas equation, calculate the pressure of oxygen gas in a cylinder with a volume of 25.00 l. the oxygen masses 4.362 kg and room temperature is at 22.5 o c. how many moles of oxygen are there and what is the pressure of oxygen in atmospheres in the cylinder according to the ideal gas law?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:50
Select the correct answer how does the heat content of the reaction change in the process of photosynthesis when a glucose molecule is formed? ca the value of is negative the value of qis positive the value of a remains constant the value of a decreases the value of equals zero e
Answers: 2


Chemistry, 22.06.2019 12:00
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2

Chemistry, 22.06.2019 12:30
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2
You know the right answer?
Using the ideal gas equation, calculate the pressure of oxygen gas in a cylinder with a volume of 25...
Questions

Mathematics, 16.07.2019 12:20

History, 16.07.2019 12:20

Health, 16.07.2019 12:20

Mathematics, 16.07.2019 12:20


Mathematics, 16.07.2019 12:20



Mathematics, 16.07.2019 12:20


Mathematics, 16.07.2019 12:20



Mathematics, 16.07.2019 12:20