
Chemistry, 09.10.2019 05:00 aleilyg2005
Given the following thermodynamic data, calculate the lattice energy of licl:
delta; h°f[licl(s)] = -409 kj/mol
δh°sublimation [li] = 161 kj/mol
bond energy [cl-cl] = 243 kj/mol
ie1 (li) = 520 kj/mol
ea1 (cl) = -349 kj/mol

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:00
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2

Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1
You know the right answer?
Given the following thermodynamic data, calculate the lattice energy of licl:
delta; h...
delta; h...
Questions





Biology, 02.07.2019 09:30



World Languages, 02.07.2019 09:30

Mathematics, 02.07.2019 09:30

History, 02.07.2019 09:30

Chemistry, 02.07.2019 09:30




Mathematics, 02.07.2019 09:30

Mathematics, 02.07.2019 09:30


History, 02.07.2019 09:30

Arts, 02.07.2019 09:30

History, 02.07.2019 09:30

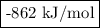
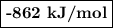 .
.

