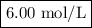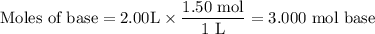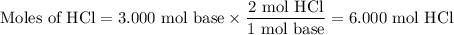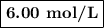Chemistry, 22.01.2020 21:31 sabahtramirez01
A1.00 l volume of hcl reacted completely with 2.00 l of 1.50 m ca(oh)2 according to the balanced chemical equation below. 2hcl + ca(oh)2 cacl2 + 2h2o what was the molarity of the hcl solution? 0.375 m 1.50 m 3.00 m 6.00 m

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 08:30
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2

Chemistry, 22.06.2019 09:00
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3

You know the right answer?
A1.00 l volume of hcl reacted completely with 2.00 l of 1.50 m ca(oh)2 according to the balanced che...
Questions

Mathematics, 20.09.2020 05:01


Mathematics, 20.09.2020 05:01

English, 20.09.2020 05:01


English, 20.09.2020 05:01


Mathematics, 20.09.2020 05:01

Biology, 20.09.2020 05:01




Mathematics, 20.09.2020 05:01




English, 20.09.2020 05:01


Spanish, 20.09.2020 05:01

Mathematics, 20.09.2020 05:01