
Chemistry, 23.11.2019 19:31 millerbe1228
Determine the enthalpy:
4nh3(g) + 7o2(g) −→ 4no2(g) + 6h2o(ℓ)
∆h◦ f species ( kj mol)
nh3(g) −46.11
no2(g) +33.2
h2o(ℓ) −285.83
1. −1899 kj/mol rxn
2. +2034.4 kj/mol rxn
3. −298.7 kj/mol rxn
4. −1766 kj/mol rxn
5. −1397.6 kj/mol rxn

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1


Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1

Chemistry, 22.06.2019 18:30
How many moles of lead are in 1.50 x 10^12 atoms of lead? could you explain the answer as well and not just give it to me i am refreshing for finals and i need to know how to do it
Answers: 3
You know the right answer?
Determine the enthalpy:
4nh3(g) + 7o2(g) −→ 4no2(g) + 6h2o(ℓ)
∆h◦ f species ( kj...
4nh3(g) + 7o2(g) −→ 4no2(g) + 6h2o(ℓ)
∆h◦ f species ( kj...
Questions


Spanish, 17.08.2021 20:30

Biology, 17.08.2021 20:30

History, 17.08.2021 20:30

Mathematics, 17.08.2021 20:30

Mathematics, 17.08.2021 20:30

English, 17.08.2021 20:30

Mathematics, 17.08.2021 20:30


English, 17.08.2021 20:30

Mathematics, 17.08.2021 20:40








Computers and Technology, 17.08.2021 20:40

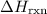 from the standard enthalpy of formation
from the standard enthalpy of formation 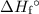 of each species.
of each species.![\Sigma \left[\Delta H_f\textdegree{}(\text{Products})]\right](/tpl/images/0388/1909/1223d.png) . Multiply the
. Multiply the ![\Sigma \left[\Delta H_f\textdegree{}(\text{Reactants})]\right](/tpl/images/0388/1909/51ea9.png) . Similarly, multiply the
. Similarly, multiply the 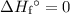 for the most stable allotrope of each elements under their standard state. For example,
for the most stable allotrope of each elements under their standard state. For example, 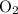 (not
(not 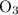 ozone) is the most stable allotrope of oxygen under STP.
ozone) is the most stable allotrope of oxygen under STP. 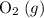 . Note the gaseous state symbol. This value is implied such that it's not provided in the question.
. Note the gaseous state symbol. This value is implied such that it's not provided in the question. ![\Sigma \left[\Delta H_f\textdegree{}(\textbf{Products})]\right = 4 \times (+33.2) + 6\times (-285.83) = -1582.18\;\text{kJ}\cdot\text{mol}^{-1}](/tpl/images/0388/1909/4e1c4.png) .
.![\Sigma \left[\Delta H_f\textdegree{}(\textbf{Reactants})]\right = 4 \times (-46.11) + 7\times 0 = -184.44 \;\text{kJ}\cdot\text{mol}^{-1}](/tpl/images/0388/1909/4a036.png) .
.![\begin{aligned} \Delta H_\text{rxn} &= \Sigma \left[\Delta H_f\textdegree{}(\textbf{Products})]\right - \Sigma \left[\Delta H_f\textdegree{}(\textbf{Reactants})]\right\\ &= -1582.18\;\text{kJ}\cdot\text{mol}^{-1} - (-184.44 \;\text{kJ}\cdot\text{mol}^{-1})\\ &= -1397.74\;\text{kJ}\cdot\text{mol}^{-1}\end{aligned}](/tpl/images/0388/1909/dcaf7.png) .
.


