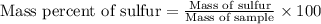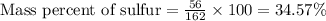Chemistry, 25.09.2019 23:00 nataliahenderso
Allicin (c6h10os2) contains 59.9 g of carbon, 21.7 g of hydrogen, 24.4 g of oxygen, and sulfur. what is the mass percent of sulfur in a 162 g sample of the compound?
a) 13.40%
b) 15.06%
c) 21.04%
d) 34.57%
e) 36.98%

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:40
Which of the following is a testable hypothesis? a. if i brush my teeth, i will get fewer cavities than if i don't brush my teeth. b. green toothpaste tastes better than blue toothpaste or red toothpaste. c. smart, careful, healthy people always brush their teeth. d. it's wrong to not brush your teeth before you have an important conversation with someone.
Answers: 1

Chemistry, 22.06.2019 01:30
Asap! how do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 1

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1
You know the right answer?
Allicin (c6h10os2) contains 59.9 g of carbon, 21.7 g of hydrogen, 24.4 g of oxygen, and sulfur. what...
Questions

Mathematics, 04.12.2020 22:30

Mathematics, 04.12.2020 22:30



Mathematics, 04.12.2020 22:30

Biology, 04.12.2020 22:30

History, 04.12.2020 22:30

Biology, 04.12.2020 22:30



Biology, 04.12.2020 22:30

Mathematics, 04.12.2020 22:30


Engineering, 04.12.2020 22:30


Spanish, 04.12.2020 22:30


Biology, 04.12.2020 22:30