
Chemistry, 16.10.2019 06:30 wafflewarriormg
Given the following balanced chemical reaction, what volume of 3.0m h2so4 is required to neutralize 60.0 ml of 0.5m naoh? be sure to include the formula and show your work for each step in the calculation.


Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Water (2510 g ) is heated until it just begins to boil. if the water absorbs 5.09×105 j of heat in the process, what was the initial temperature of the water?
Answers: 3

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1


Chemistry, 22.06.2019 20:30
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3
You know the right answer?
Given the following balanced chemical reaction, what volume of 3.0m h2so4 is required to neutralize...
Questions

Health, 02.12.2019 01:31




Mathematics, 02.12.2019 01:31


Geography, 02.12.2019 01:31


English, 02.12.2019 01:31

History, 02.12.2019 01:31





English, 02.12.2019 01:31





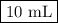

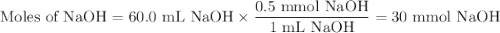
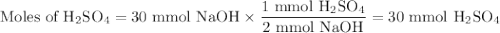
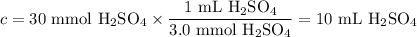
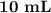 H₂SO₄.
H₂SO₄.

