
Chemistry, 24.09.2019 05:40 kimberly01262016
Achemical process dissolves 500 milligrams of iron oxide every 20 minutes. how long would it take this reaction to dissolve 2 lbs of iron oxide?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Drive down any three characteristic of modern periodic table
Answers: 1


Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 14:00
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2
You know the right answer?
Achemical process dissolves 500 milligrams of iron oxide every 20 minutes. how long would it take th...
Questions


Mathematics, 29.08.2019 07:00


Social Studies, 29.08.2019 07:00

Mathematics, 29.08.2019 07:00




Mathematics, 29.08.2019 07:00

Mathematics, 29.08.2019 07:00

English, 29.08.2019 07:00

History, 29.08.2019 07:00





Mathematics, 29.08.2019 07:00


Mathematics, 29.08.2019 07:00

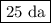
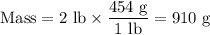
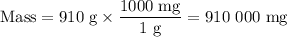
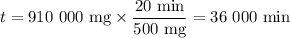
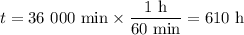
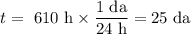
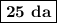 to dissolve the iron oxide.
to dissolve the iron oxide.


