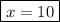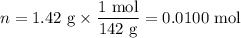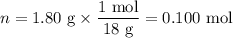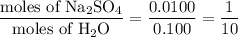Chemistry, 20.01.2020 08:31 mercedesamatap21hx0
When a 3.22 g sample of an unknown hydrate of sodium sulfate, na2so4 . x h2o (s), is heated, h2o (molar mass 18 g) is driven off. the mass of the anhydrous na2so4 (s) (molar mass 142 g) that remains is 1.42g. the value of x in the hydrate is

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:10
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Chemistry, 22.06.2019 08:30
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2
You know the right answer?
When a 3.22 g sample of an unknown hydrate of sodium sulfate, na2so4 . x h2o (s), is heated, h2o (mo...
Questions

Mathematics, 20.05.2021 16:30

Mathematics, 20.05.2021 16:30

Health, 20.05.2021 16:30

History, 20.05.2021 16:30

Computers and Technology, 20.05.2021 16:30



Chemistry, 20.05.2021 16:30


Mathematics, 20.05.2021 16:30

Mathematics, 20.05.2021 16:30

Mathematics, 20.05.2021 16:30

Mathematics, 20.05.2021 16:30

Computers and Technology, 20.05.2021 16:30

Mathematics, 20.05.2021 16:30

Mathematics, 20.05.2021 16:30



Mathematics, 20.05.2021 16:30

History, 20.05.2021 16:30