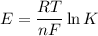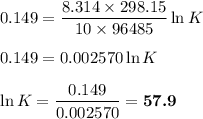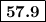Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
In the 1800s, one of the statements in john dalton's atomic theory was that atoms are indivisible. later experimental evidence led to the discovery of subatomic particles such as neutrons, electrons, and protons. what happened to the indivisible atom part of dalton's atomic theory, and why?
Answers: 3

Chemistry, 22.06.2019 07:30
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3


Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1
You know the right answer?
From standard reduction potentials, calculate the equilibrium constant at 25 ∘c for the reaction 2mn...
Questions


Mathematics, 19.08.2021 20:50

Mathematics, 19.08.2021 20:50

Mathematics, 19.08.2021 20:50

Mathematics, 19.08.2021 20:50

English, 19.08.2021 20:50

Business, 19.08.2021 20:50

Mathematics, 19.08.2021 20:50





Mathematics, 19.08.2021 20:50


Mathematics, 19.08.2021 20:50



Chemistry, 19.08.2021 20:50

Biology, 19.08.2021 20:50

Social Studies, 19.08.2021 20:50