Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3

Chemistry, 22.06.2019 23:30
With the largest atoms and the smallest number of valence electrons and with the smallest atoms and the greatest number of valence electrons are the most reactive. a. nonmetals; metals b. nonmetals; transition elements c. transition elements; metals d. metals; nonmetals
Answers: 3
You know the right answer?
Phosphorus pentachloride decomposes to phosphorus trichloride at high temperatures according to the...
Questions


Mathematics, 11.07.2019 12:00

History, 11.07.2019 12:00

History, 11.07.2019 12:00



Mathematics, 11.07.2019 12:00

English, 11.07.2019 12:00

Computers and Technology, 11.07.2019 12:00

History, 11.07.2019 12:00

Mathematics, 11.07.2019 12:00


Mathematics, 11.07.2019 12:00

History, 11.07.2019 12:00


Arts, 11.07.2019 12:00

Health, 11.07.2019 12:00

Mathematics, 11.07.2019 12:00

Mathematics, 11.07.2019 12:00

![\boxed{\text{[PCl$_{5}$] = 0.0077 mol/L; [PCl$_{3}$] = [Cl$_{2}$] = 0.117 mol/L}}](/tpl/images/0449/9090/348ff.png)
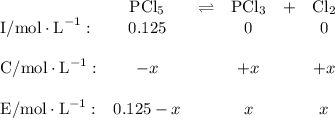
![K_{\text{c}} = \dfrac{\text{[PCl$_3$][Cl$_2$]}}{\text{[PCl$_5$]}} = \dfrac{x^{2}}{0.125-x} = 1.80](/tpl/images/0449/9090/2dff3.png)
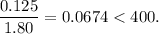
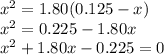
![\boxed{\textbf{[PCl$_{5}$] = 0.0077 mol/L; [PCl$_{3}$] = [Cl$_{2}$] = 0.117 mol/L}}](/tpl/images/0449/9090/a2840.png)