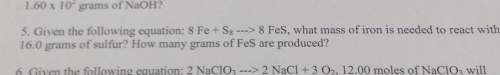Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 01:30
Select the correct answer from each drop-down menu. to make a table of the elements, dmitri mendeleev sorted the elements according to their . he then split the list of elements into several columns so that elements beside each other had similar .
Answers: 2

Chemistry, 23.06.2019 03:30
The semi-conductors on the periodic table are classified as
Answers: 1

Chemistry, 23.06.2019 04:10
Which of the following is described by the equation h2o(s)+ heat=h2o(i) a freezing melting condensing evaporating
Answers: 2

You know the right answer?
5. given the following equation: 8 fe s8 8 fes, what mass of iron is needed to react with16.0 grams...
Questions

Social Studies, 30.06.2019 19:30

Spanish, 30.06.2019 19:30

English, 30.06.2019 19:30


Mathematics, 30.06.2019 19:30


Spanish, 30.06.2019 19:30

Advanced Placement (AP), 30.06.2019 19:30


English, 30.06.2019 19:30

Mathematics, 30.06.2019 19:30

History, 30.06.2019 19:30


Mathematics, 30.06.2019 19:30





Chemistry, 30.06.2019 19:30