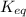Chemistry, 03.01.2020 17:31 HalpMehPlz
Which of the following is true for the equilibrium constant of a reaction?
it is a ratio of coefficients of reactants to products.
it has a different value at different temperatures.
it is represented by the symbol h.
its value is always less than 1.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
When the following equation is balanced using the smallest possible integers, what is the coefficent of oxygen gas? c7h16o(g) + o2(g) → co2(g) + h2o(g) -1 -5 -8 -16 -21
Answers: 3

Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2

Chemistry, 23.06.2019 02:00
To calculate the molarity of a solution, you need to know the moles of solute and the
Answers: 2

Chemistry, 23.06.2019 04:20
The reaction below shows a system in equilibrium. how would a decrease in temperature affect this reaction? a. the rate of formation of the gases would increase. b. the equilibrium of the reaction would shift to the left. c. the equilibrium would shift to cause the gases to sublime into solids. d. the chemicals on the left would quickly form the chemical on the right.
Answers: 1
You know the right answer?
Which of the following is true for the equilibrium constant of a reaction?
it is a rati...
it is a rati...
Questions


Mathematics, 06.02.2021 05:10

Computers and Technology, 06.02.2021 05:10








Mathematics, 06.02.2021 05:10


Arts, 06.02.2021 05:10

Mathematics, 06.02.2021 05:10

Mathematics, 06.02.2021 05:10

Mathematics, 06.02.2021 05:10




Mathematics, 06.02.2021 05:10