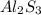Chemistry, 20.10.2019 09:50 strange5eyes
The formula of aluminium sulphide is al₂s₃. explain why the formula has a ratio of two aluminium ions for every three sulphide ions.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3

Chemistry, 22.06.2019 13:30
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1

Chemistry, 22.06.2019 15:30
Which of the following are correct values for the ideal gas laws constant r
Answers: 1

Chemistry, 23.06.2019 03:40
The following questions a24 - a26 relate to 100 ml of 0.0150 m solution of benzoic acid (c6h3cooh). ka(c6h3cooh) = 6.4 x 10^-5. what is the ph of the solution after the addition of 1 x 10^-3 moles of naoh? you may assume no volume change to the solution upon addition of the naoh.
Answers: 2
You know the right answer?
The formula of aluminium sulphide is al₂s₃. explain why the formula has a ratio of two aluminium ion...
Questions

Spanish, 22.04.2020 03:58

English, 22.04.2020 03:58


Mathematics, 22.04.2020 03:58


Mathematics, 22.04.2020 03:58


Computers and Technology, 22.04.2020 03:58







Spanish, 22.04.2020 03:58





History, 22.04.2020 03:58