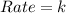Chemistry, 05.02.2020 02:50 damienlopezram
What is the overall order of reaction for this rate law: rate = k ? a. zero b. first c. second d. third

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
The graph above shows how the price of cell phones varies with the demand quantity. the equilibrium price for cell phones is where both supply and demand quantities equal $100, 5,000 5,000, $100
Answers: 2

Chemistry, 22.06.2019 08:30
Which of the following would have less momentum than a 52 kg cheetah running at 10 m/s?
Answers: 2

Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2

Chemistry, 22.06.2019 23:30
The sum of the oxidation numbers in a neutral compound is always
Answers: 2
You know the right answer?
What is the overall order of reaction for this rate law: rate = k ? a. zero b. first c. second d....
Questions




History, 06.06.2020 05:03

Mathematics, 06.06.2020 05:03


Mathematics, 06.06.2020 05:03



Mathematics, 06.06.2020 05:03



Advanced Placement (AP), 06.06.2020 05:03




Mathematics, 06.06.2020 05:03

Spanish, 06.06.2020 05:03

English, 06.06.2020 05:03