
Chemistry, 02.02.2020 12:49 paigefields2578
Formic acid, hcooh, is a weak acid present in the venom of red-ants. at equilibrium, [hcooh] = 2.00 m, [hcoo− ] = 4.0 × 10− 1 m, and [h3o+ ] = 9.0 × 10− 4 m.
a. write the equilibrium expression for the ionization

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Iwll give extra points to who gets this for ! what type of reaction is this? ?
Answers: 2

Chemistry, 22.06.2019 09:30
The chart shows the bid provided by four contractors to complete a job. which contractor is the most cost-effective?
Answers: 3

Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 23.06.2019 01:30
Astudent states that 9.0 g of baking soda will form an unsaturated solution in 100 g of water. what do you need to know to decide whether this statement is correct? a. the temperature of the water and the molar mass of baking soda b. the percent by volume of the solution and the solubility of baking soda c. the temperature of the water and the solubility of baking soda at that temperature
Answers: 1
You know the right answer?
Formic acid, hcooh, is a weak acid present in the venom of red-ants. at equilibrium, [hcooh] = 2.00...
Questions


Mathematics, 23.06.2019 05:30



Mathematics, 23.06.2019 05:30




Biology, 23.06.2019 05:30


Mathematics, 23.06.2019 05:30








Mathematics, 23.06.2019 05:30

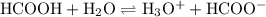
![K_{eq} = \dfrac{[\text{Products}]}{[\text{Reactants}]}](/tpl/images/0492/9894/57030.png)
![K_{eq} = \dfrac{[\text{HCOO}^{-}][\text{H}_{3}\text{O}^{+}]}{[\text{HCOOH}]}](/tpl/images/0492/9894/43500.png)


